Contents
Nānā Bicarbonate
Ka wehewehe o ka bicarbonates
ka ion bicarbonates (HC03-) aia i loko o ke koko: hana nui lākou i ka hooponopono pH. ʻO lākou ka "buffer" nui o ke kino.
No laila, pili pono ko lākou ʻike i ke koko i ka pH. ʻO ia ka mea nui o nā puʻupaʻa e hoʻoponopono i ka neʻe ʻana o nā bicarbonates koko, e hāpai ana i ko lākou paʻa ʻana a i ʻole excretion.
No ka hoʻoponopono ʻana i ka pH, ʻo ka bicarbonate ion HCO3- hui me ka H ion+ e hāʻawi i ka wai a me CO2. ʻO ke kaumaha en CO2 i ke koko koko (Pa CO2), a i ʻole capnia, a i ʻole ke kaomi ʻāpana i hana ʻia e ka CO2 i hoʻoheheʻe ʻia i loko o ke koko aʻa, no laila he hōʻailona hoʻi ia o ke koena acid-base. Ua ana ʻia i ka wā o ka nānā ʻana i nā kinoea koko.
He mea maʻamau nā ion bicarbonate: ke piʻi aʻe ko lākou ʻike, piʻi pū ka pH. ʻO ka mea ʻē aʻe, i ka emi ʻana o kā lākou neʻe ʻana, lilo ka pH i acidic.
I ke kanaka olakino, paʻa loa ka pH koko: 7,40 ± 0,02. ʻAʻole pono ia e hāʻule ma lalo o 6,6 a ʻaʻole hoʻi e piʻi ma luna o 7,7, ʻaʻole kūpono i ke ola.
No ke aha e hana ʻia ai kahi ʻike bicarbonate?
ʻO ke ʻano o nā iona bicarbonate e hiki ai ke loiloi i ke koena acid-base o ke koko. Hana ʻia ia i ka manawa like me ka nānā ʻana i nā kinoea koko, ke kānalua nei ke kauka i ka loaʻa ʻana o kahi imbalance acid-base (acidosis a i ʻole alkalosis). Hiki paha kēia ma ke alo o kekahi mau hōʻailona, e like me:
- ke kūlana i hoʻololi ʻia o ka ʻike
- hypotension, haʻahaʻa cardiac puka
- nā pilikia hanu (hypo- or hyperventilation).
- A i ʻole ma nā kūlana koʻikoʻi e like me ka hoʻoheheʻe ʻana a i ʻole ka nalo ʻana o ka mimi a i ʻole nā pilikia electrolyte.
ʻO ka loiloi o ka bicarbonates
ʻO ka hoʻāʻo koko he laʻana o ke koko venous, maʻamau ma ka ʻāpana o ka kuʻekuʻe. ʻAʻole pono ka hoʻomākaukau.
He aha nā hopena e hiki iā mākou ke manaʻo mai ka nānā ʻana i nā bicarbonates?
ʻO ka nānā ʻana i hiki ke ʻike i ke alo o waikawa ai ole ia, he alkalosis. E ʻae ke ana pH iā ʻoe e ʻike inā loaʻa ka hyperacidemia (i wehewehe ʻia ma ke ʻano he pH ma lalo o 7,35) a i ʻole hyperalcalemia (pH waiwai ma luna o 7,45).
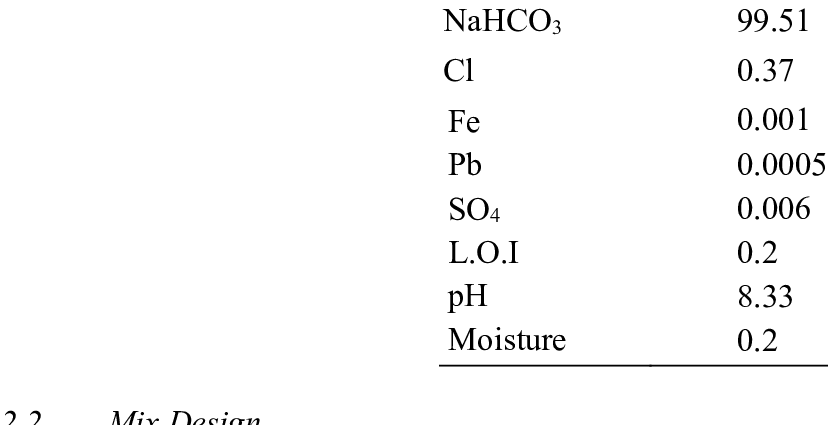
Ana o na iona bicarbonate a me PaCO2 a laila e hiki ai ke hoʻoholo inā no ka metabolic kumu (abnormality of bicarbonates) a i ʻole respiratory (abnormality of PaCO).2). ʻO nā kumukūʻai maʻamau no ka bicarbonates ma waena o 22 a me 27 mmol / l (millimoles no ka lita).
ʻO ka emi ʻana o ka neʻe ʻana o nā ion bicarbonate ma lalo o nā waiwai maʻamau acidosis metabolic. Hoʻopili ʻia ka acidosis i ka nui o nā ion H +. I ka hihia o ka metabolic acidosis, e emi ana ka nui o nā ion bicarbonate (pH <7,35). I ka acidosis hanu, ʻo ia ka piʻi ʻana o ke kaomi hapa o CO2 ʻo ia ke kuleana no ka hoʻonui ʻana i nā ion H +.
ʻO ka metabolic acidosis ma muli o kekahi mau mea ʻē aʻe, i ka nalowale ʻole o nā bicarbonates ma muli o ka maʻi maʻi a i ʻole ka infusion saline physiological.
ʻO ka mea ʻē aʻe, ʻo ka hoʻonui ʻana i ka ʻike o nā iona carbonate e alakaʻi i kahi alkalosis metabolic (pH> 7,45). Hiki ke loaʻa i ka hopena o ka hoʻohana nui ʻana i ka bicarbonates, ka luaʻi nui a i ʻole ka nalowale o ka potassium (diuretics, diarrhea, luaʻi). Hiki ke komo pū ka Hyperaldosteronism (hypersecretion of aldosterone).
ʻO ka alkalosis respiratory, ma kāna ʻāpana, pili i ka emi kaʻawale o ke kaomi ʻāpana o CO2.
Heluhelu pū kekahi: ʻO nā mea a pau e pili ana i ka hypotension |